On Thursday (24/8/2022), Faculty of Pharmacy UNAIR collaborated with The 3rd International Summer School AIPHE 2022 to hold a webinar for the 7th day. In a series of events which were held offline at Hall 1st floor of Nanizar Zaman Joenoes Building and online on the APTFI digital channel, 3 speakers were invited to discuss various matters regarding pharmaceuticals.
The first speaker discussed "New Perspectives in the Development of Herbal Medicine Towards Excellence" with Raymond R. Tjandrawinata, Ph.D. as resource person. He stressed that original products such as phytopharmaca must continue to be preserved and developed because the pharmacological effects provided can be very useful.
"Starting from the original Indonesian biodiversity, then research by scientists who can provide medical evidence for pre-clinical and clinical trials. The clinical trials themselves will be carried out by doctors from Indonesia so that data on the mechanism of action of the drug can then be obtained," he said.
The second speaker, Joko Kawiyanto, brought the theme "EXTRACTION TECHNOLOGY: Laboratory Scale to Mass Production". In his presentation, Joko revealed that there are several things that must be considered in moving from laboratory-scale extraction to large-scale extraction because the difference in numbers is quite significant.
"Several critical points that need to be considered when we make changes from laboratory scale to mass scale are regarding the method used, the type of solvent or solvent, the characteristics of the extracted simplicia itself, and the final product," he explained.
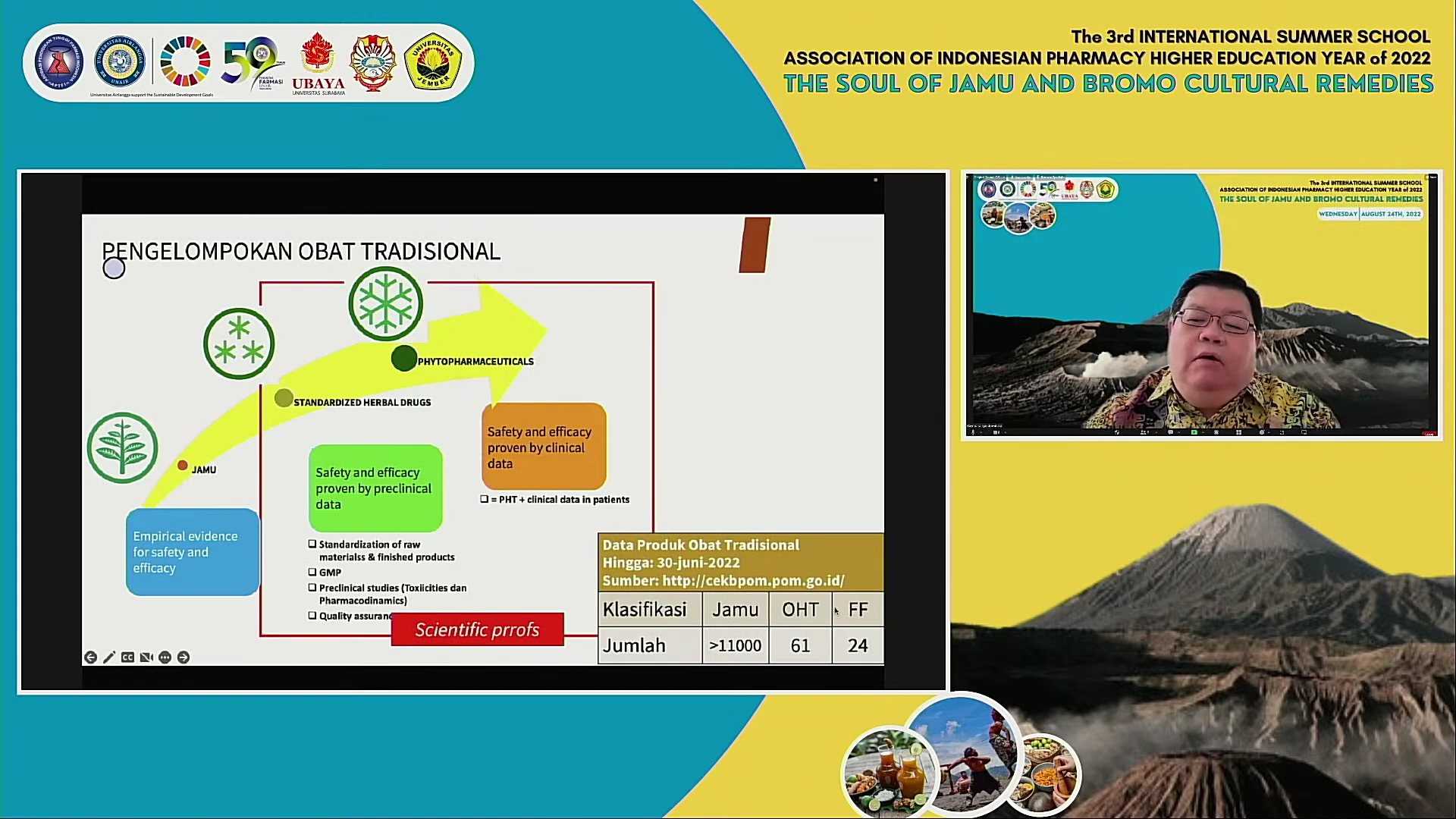
The last speaker came from the Faculty of Pharmacy UNAIR itself, namely Dr.apt.Budiastuti, MS. with the title "QC (Quality Control) on Herbal Extracts". He explained that before being processed into medicinal preparations, herbal raw materials first go through various complex processes, such as extraction, and then separate based on their fractions according to their solubility.
"Quality control is not only limited to laboratory activities, but also all decisions regarding drug quality. Drug quality is very important because correct pharmacological activity also depends on drug quality," he explained.
The series of events that day started with a brief presentation of the material and ended with a discussion session. At the end of the event, an online certificate submission session and reading of the agenda for the next day were held.
Reporter and Editor: Leivina (2021)








